Mechanisms of sensory and motor circuit development
The precise formation and connection of neuronal networks is essential for the proper function of the nervous system, including sensory and motor circuits. We aim to understand the molecular and cellular mechanisms that regulate neuronal connectivity and to leverage this knowledge to develop regenerative therapeutic strategies for visual pathologies, including glaucoma.
Presentation
Our team investigates three essential stages in the formation of neuronal circuits during development: (1) neuronal migration, which enables newly generated neurons to reach their final position within the nervous system; (2) axonal guidance, which allows each neuron to connect with appropriate synaptic partners; and (3) axonal pruning, which refines neuronal connections by eliminating incorrect or misplaced synapses. These three critical steps of neuronal development are regulated by a set of chemotropic molecules, also known as guidance molecules. Interestingly, these molecules not only influence neuronal development but also control the behavior of other cell types, such as endothelial cells that form the vasculature. Our projects aim to identify the intracellular mechanisms that enable neurons to detect and interpret these environmental molecules during each of these three developmental stages. Understanding these molecular mechanisms not only advances our knowledge of neuronal circuit formation but also allows us to design and test potential therapeutic strategies targeting neurodegenerative diseases, including glaucoma.
- Signal transduction downstream of guidance molecules
One of the first intracellular events triggered by axonal guidance molecules is the change in concentration of signaling molecules known as second messengers. Surprisingly, these cellular signals are shared by nearly all signaling pathways and thus influence the full range of cellular behaviors. To ensure that second messengers specifically regulate the cellular events they control, these molecules are finely compartmentalized within distinct cellular domains. Each signal, restricted to a given compartment, will regulate a single signaling pathway and a single downstream cellular behavior. We seek to identify the cellular domains that contribute to the regulation of cell migration, axonal guidance, and pruning. Each identified compartment is then characterized to reveal the cellular events it controls in developing neurons, as well as in endothelial cells. For these studies, we employ cutting-edge microscopy approaches, molecular tools that manipulate cellular signals with subcellular resolution, and optogenetic approaches to generate light-initiated second messenger signals with controlled subcellular localization and temporal characteristics.
- Cytoskeletal Remodeling Induced by Guidance Molecules
Once environmental molecules are detected and decoded by cells, various cellular events are initiated, including profound remodeling of the cellular skeleton (cytoskeleton), leading to morphological changes in the cell. This remodeling is essential for the wiring of neuronal circuits, and its dysfunction underlies a range of neurodevelopmental disorders. We study the role of a family of proteins implicated in human neurological diseases in the establishment of neuronal connectivity and axonal navigation. Our goal is to identify the molecular and cellular processes controlled by these proteins in developing axons and to correlate them with the mechanisms associated with neurological diseases linked to these proteins.
- Development of an In Vitro Glaucoma Model
It is conceivable that the cellular and molecular mechanisms uncovered by our studies could be harnessed to design regenerative strategies in the context of neurodegenerative diseases, thereby developing methods to repair a damaged nervous system. However, to test these approaches, an effective disease model is required. An in vitro model is particularly valuable as it allows for the parallel testing of numerous factors. Currently, such a model does not exist for glaucoma, a blinding disease characterized by the loss of connection between the retina and the brain. We have undertaken the development of an in vitro glaucoma model using retinal organoids, a method that closely mimics the characteristics of the human retina.
By deciphering the subcellular codes of second messengers and characterizing the role of cytoskeletal-associated proteins in the wiring of visual and motor circuits, our projects will deepen our understanding of the molecular mechanisms underlying neuronal connectivity. This work will also shed new light on the etiology of neurodevelopmental and neurodegenerative disorders and provide therapeutic targets or tools to preserve or (re)establish functional connectivity under pathological conditions.
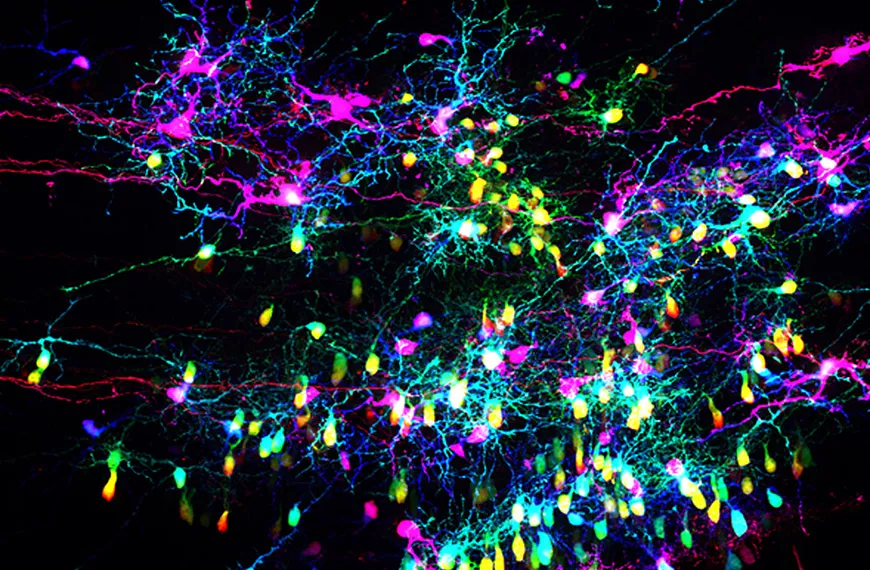
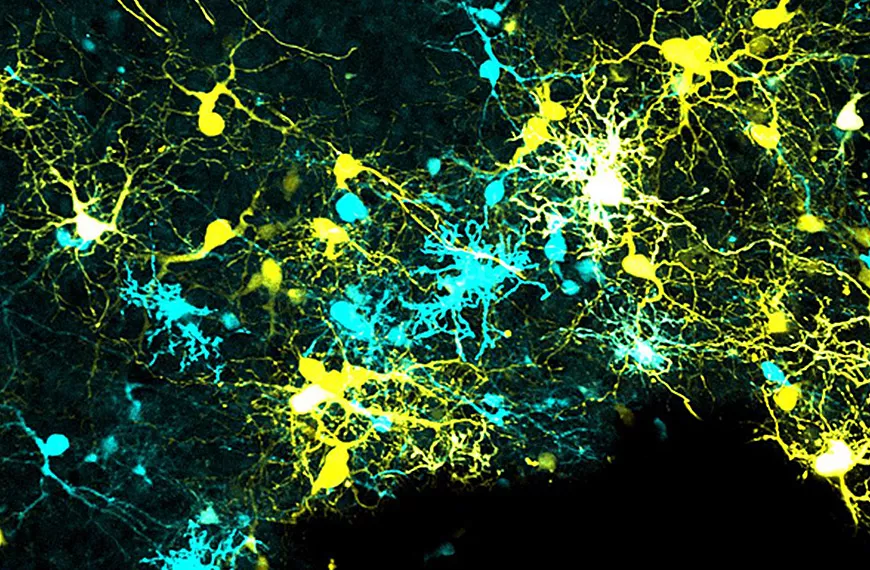
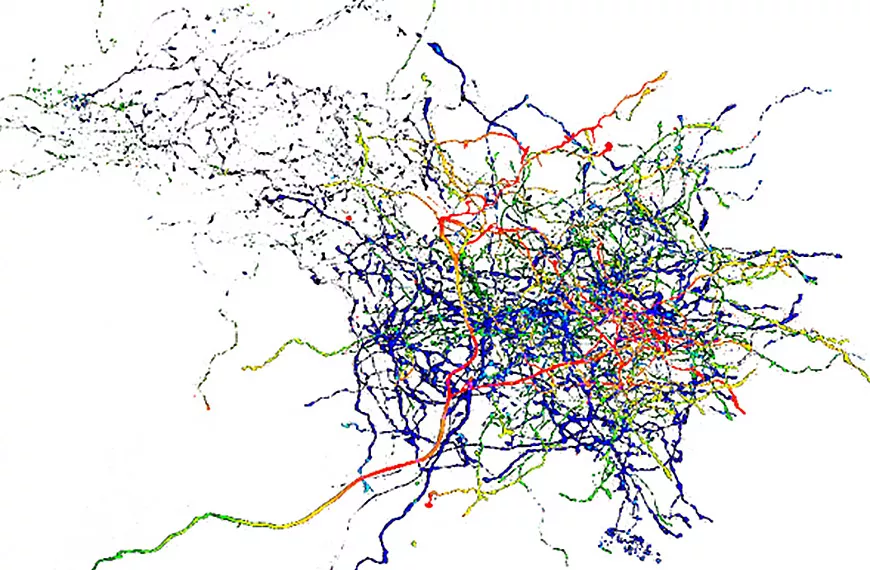
Research areas
- Development of central nervous system connectivity
- Cellular signaling controlling cell and axonal motility
- Axonal cytoskeleton remodeling
- Neuronal migration
- Vascular development
- Neurodegenerative pathologies
- Regenerative therapeutic strategies
Team members
Scientific publications
Below you will find the latest scientific publications in this field: Mechanisms of sensory and motor circuit development.













