Actualités
Événements, portraits de chercheurs, avancées marquantes, ou encore récompenses et distinctions : vivez au rythme de l’Institut de la Vision en suivant ses actualités.
> Toutes les actualités
Communication
Le rapport d'activité 2024 de l'Institut de la Vision est en ligne
L'Institut de la Vision dévoile son rapport d'activité 2024, une année d’accélération pour l’innovation et la recherche.
Lire l'article
Générosité
« Ciel, ma rétine ! » : un livre coup de cœur au profit de l’Institut de la Vision
Depuis de nombreuses années, Marie de Ruffey accompagne et soutient l’Institut de la Vision dans sa mission pour améliorer…
Lire l'article
Communication
La Fédération des Aveugles et Amblyopes de France, un engagement indéfectible pour la recherche
Depuis toujours, la Fédération des Aveugles et Amblyopes de France œuvre pour l’autonomie, l’inclusion et la…
Lire l'article
Évènement
Les 41 èmes Rencontres Nationales de l’IRRP
L’association Information Recherche Rétinite Pigmentaire est heureuse de vous annoncer les 41èmes Rencontres Nationales de…
Lire l'article
Séminaire
L'importance de la science des données dans les contextes cliniques et non cliniques : Paradigmes avancés de traitement des données et des signaux
Invité par Chiara Boscarino et Ulisse Ferrari, le Pr Riccardo Barbieri (Département d'électronique, d'information et de…
Lire l'article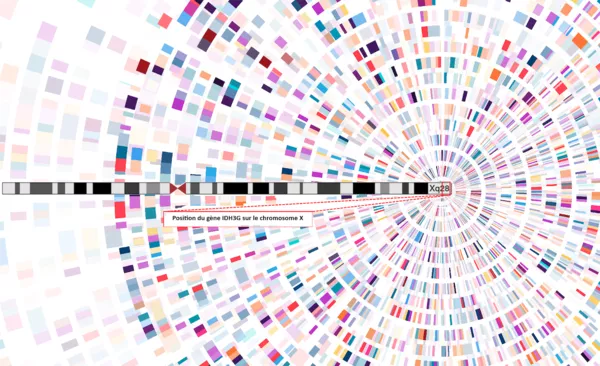
Recherche
Découverte du gène IDH3G : une avancée pour la rétinite pigmentaire liée à l’X
À l’Institut de la Vision, l’équipe dirigée par Isabelle Audo et Christina Zeitz a identifié un nouveau gène impliqué dans…
Lire l'article
Séminaire
Microstimulation électrique pour sonder l'activité à l'échelle du cerveau grâce à l'IRMf
Invité par Florian Fallegger, le Dr Sjoerd R. Murris (Institut néerlandais de neuroscience) interviendra le…
Lire l'article
Séminaire
Cellules microgliales dans le cerveau humain : motilités, phénotypes et interactions neuronales distincts dans le développement et l'épilepsie
Invité par Florian Fallegger, le Dr Giampaolo Milior (Chercheur-Centre d'Imagerie Moléculaire, Institut Jacob, Université…
Lire l'article
Recherche
Et si le venin de vipère permettait de lutter contre la DMLA et la rétinopathie diabétique ?
Le Dr Xavier Guillonneau et son équipe étudient les propriétés anti-angiogéniques de la lébécétine, une molécule extraite…
Lire l'article
Évènement | Recherche
Lutèce Dynamics, spin-off qui révolutionne l’imagerie biomédicale
Lutèce Dynamics est une start-up issue des travaux de l’Institut de la Vision et de l’Institut Langevin. Créée pour…
Lire l'article